HEK293T cells, derived from human embryonic kidney cells, are widely used in gene therapy research due to their transfection efficiency and capability to produce different types of viral vectors. These cells are especially suitable for generating lentiviral (LV) vectors, which makes them a valuable tool for CAR T cell therapy production and other advanced therapeutic applications.
Lentiviral Vectors and CAR T Therapy
Lentiviral vectors are derived from the HIV-1 virus and are particularly valued for their ability to integrate into the host genome, enabling long-term expression of therapeutic genes. In CAR T therapy, LV vectors are used to modify a patient’s T cells to express chimeric antigen receptors (CARs) that target cancer cells [1, 2]. LV vectors are uniquely suited for CAR T therapy because they enable stable, long-lasting expression of CARs, which is crucial for the sustained anti-tumor activity of modified T cells.
Other Viral Vectors Produced in HEK293T Cells
In addition to LV vectors, HEK293T cells are also used to produce adenoviral (Ad) and adeno-associated viral (AAV) vectors. Ad vectors are known for their high transduction efficiency, while AAV vectors are prized for their low immunogenicity and long-term expression in non-dividing cells. Each of these vectors requires specific modifications to the viral genome to ensure safety and efficacy in therapeutic applications.
Future Prospects
As gene therapy evolves, the role of HEK293T cells in producing viral vectors will continue to grow, particularly in developing more efficient and targeted treatments like CAR T therapy. Future innovations may include enhanced vector safety, better targeting capabilities, and integration with emerging technologies like CRISPR. This progress will further expand the potential of personalized medicine, offering new hope for treating complex diseases.
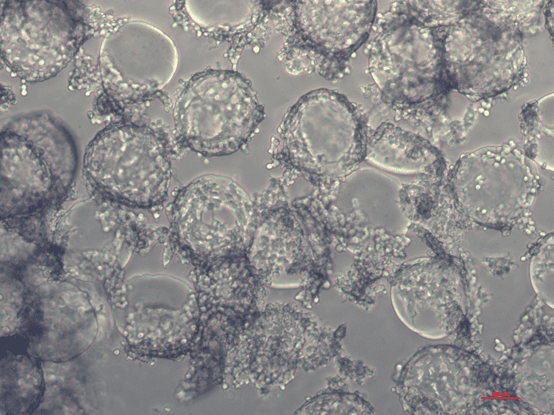
Scinus Cell Expansion is currently optimizing the conditions for large-scale HEK293T culture for CAR-T therapy production. The picture below shows HEK293T cells on dissolvable microcarriers. In the second edition of our new ‘Ask the Expert’ series, our PhD student Javier Olmos Becerra will share his experience on specific conditions for HEK293T culture, using the SCINUS bioreactor, and how he fine-tunes this process to optimize cell culture. You can also read his poster on ‘optimal conditions for HEK293T cell expansion’ in the publication section of our website. Stay tuned!
References
[1] Mhaidly, Rana, and Els Verhoeyen. “The future: in vivo CAR T cell gene therapy.” Molecular Therapy4 (2019): 707-709.
[2] Michels, Kathryn R., et al. “Preclinical proof of concept for VivoVec, a lentiviral-based platform for in vivo CAR T-cell engineering.” Journal for immunotherapy of cancer3 (2023).
SCINUS is optimizing the conditions for large-scale HEK293T culture for CAR-T therapy production within the SCINUS bioreactor NG.
