Organoids have emerged as a revolutionary tool in drug discovery and clinical practice. These miniature 3D structures, cultivated from stem cells or differentiated cells, mimic the architecture and function of organs, offering a significant advancement over traditional cell culture methods. Organoids have demonstrated remarkable potential in disease modeling, drug screening, and personalized medicine. For example, researchers have used brain organoids to study neurological disorders such as Alzheimer’s disease and screen potential therapeutic compounds [1]. Similarly, intestinal organoids have been employed to investigate gastrointestinal diseases and evaluate drug efficacy [2].
Unlike conventional 2D cell cultures, organoids can replicate the complexity and heterogeneity of human tissues more accurately [3]. However, maintaining the structure and functionality of organoids over time requires intricate culture conditions, which limit scalability and hinder widespread adoption in drug discovery and clinical settings. Bioreactors, with their precise control over culture parameters such as oxygen levels, nutrient supply, and mechanical stimulation, promote the growth and maturation of organoids [4]. This approach has the potential to standardize organoid production, improve reproducibility, and scale up for high-throughput applications. Despite these advances, technical challenges in spatiotemporal monitoring of single cells within the complex organoid structure remain.
The challenges in reducing inter- and intra-batch variability depends on the ongoing technological advancements of sensitive tools. Current single-cell RNA-sequencing of dissociated organoids fails to capture spatial information. While spatial transcriptomics integrates histological tissue sectioning with RNA-sequencing, this is not yet at single-cell level. Furthermore, advances in 3D microscopy permit complex 3D spatial mapping, but this technique is relatively low-throughout [5].
Looking ahead, the importance of organoids in biotech is expected to continue growing. Advances in technology, such as microfluidic systems and organ-on-a-chip platforms, will further enhance the functionality and relevance of organoid models. Moreover, ongoing efforts to improve culture techniques and reduce variability will broaden the utility of organoids in drug development and personalized medicine. SCINUS is actively developing reproducible and standardized protocols for the culture of clinically relevant organoid cultures for drug screening and disease modeling. Please contact us if you have any questions regarding our current protocols for organoid culture within the SCINUS bioreactor NG.
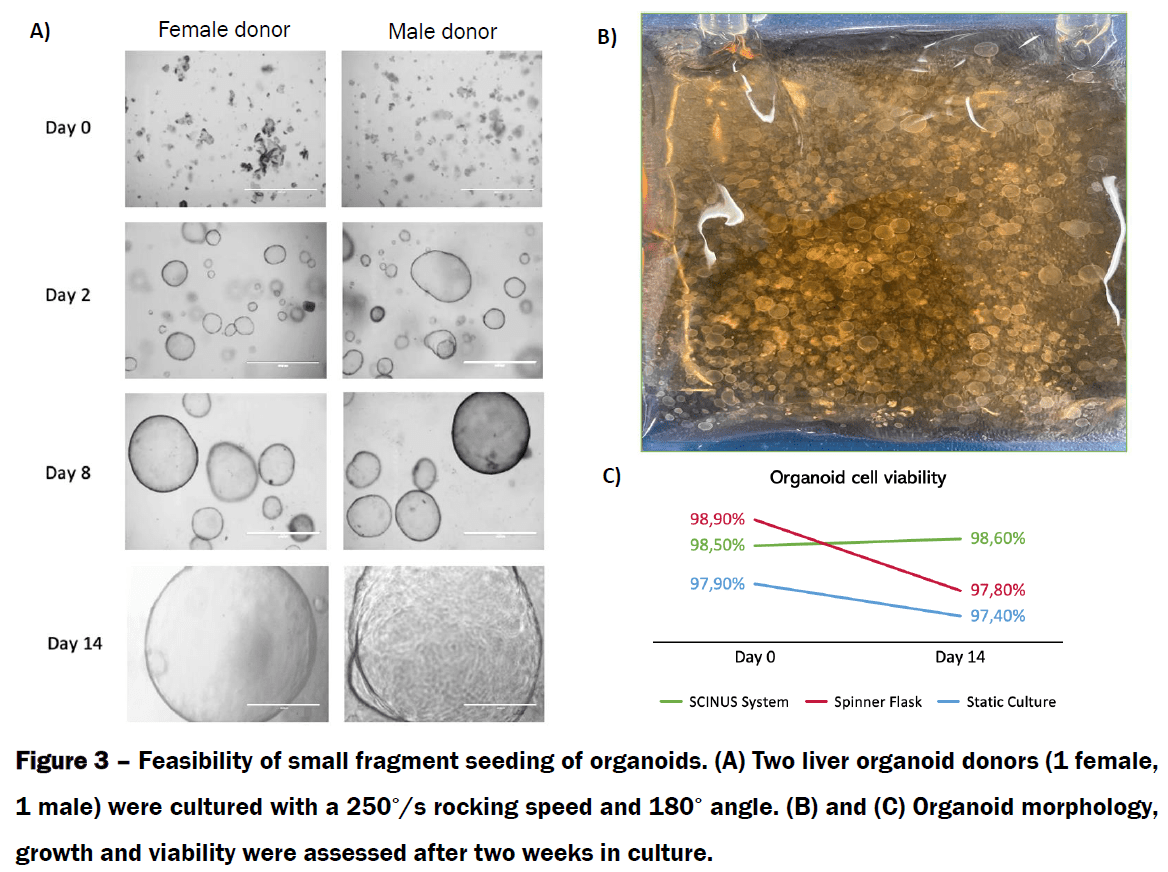
References
[1] Bi, Feng-Chen, et al. “Optimization of cerebral organoids: a more qualified model for Alzheimer’s disease research.” Translational Neurodegeneration 10 (2021): 1-13.
[2] Yoshida, Shinpei, et al. “Generation of intestinal organoids derived from human pluripotent stem cells for drug testing.” Scientific Reports 10.1 (2020): 5989.
[3] Silva-Pedrosa, Rita, António José Salgado, and Pedro Eduardo Ferreira. “Revolutionizing disease Modeling: the emergence of organoids in cellular systems.” Cells 12.6 (2023): 930.
[4] Licata, Joseph P., et al. “Bioreactor technologies for enhanced organoid culture.” International Journal of Molecular Sciences 24.14 (2023): 11427.
[5] Matthys, Oriane B., Ana C. Silva, and Todd C. McDevitt. “Engineering human organoid development ex vivo—challenges and opportunities.” Current Opinion in Biomedical Engineering 13 (2020): 160-167.
SCINUS is actively developing reproducible and standardized protocols for the culture of clinically relevant organoid cultures for drug screening and disease modeling.
